Idelalisib (CAL-101)
别名: GS-1101
中文名称:艾代拉里斯,艾代拉利司
目录号:S2226 Purity: 99.93%
Idelalisib (CAL-101)是选择性p110δ抑制剂,在无细胞试验中IC50为 2.5 nM;对 p110δ 表现出的选择性是对 p110α/β/γ 的 40 到 300 倍,对p110δ的选择性是对 C2β,hVPS34,DNA-PK 和 mTOR的400到4000倍。Idelalisib 还可诱导自噬。
CAS: 870281-82-6
客户使用Selleck的Idelalisib (CAL-101)发表文献276篇
- Cancer Cell, 2023 41(6):1103-1117.e12
- Cancer Cell, 2022 S1535-6108(22)00312-9
- Sci Immunol, 2022 7(75):eadd4906
- Blood, 2022 blood.2021014304
- Cell, 2020 S0092-8674(20)31394-5
- Nat Mater, 2019 18(8):892-904
- Gastroenterology, 2019 157(6):1615-1629
- Blood, 2019 134(21):1821-1831
- Cell, 2018 173(2):470-484
- Lancet Oncol, 2018 19(4):486-496
- Cancer Cell, 2018 34(6):982-995
- Nature, 2017 546(7657):302-306
- Nature, 2017 552(7683):121-125
- Nature, 2017 23;542(7642):489-493.
- Blood, 2017 5;129(1):88-99
- Blood, 2017 27;130(4):501-513
- Blood, 2017 1;129(22):3000-3008
- Nature, 2016 539(7629):437-442
- Ann Oncol, 2016 27(6):1123-8
- Blood, 2016 127(25):3192-201
- Blood, 2016 128(4):574-83
- Nature, 2015 517(7535):460-5
- Nature, 2015 22;517(7535):460-5.
- Blood, 2015 126(11):1336-45
- Blood, 2015 10.1182/blood-2014-12-618470
- Blood, 2015 10.1182/blood-2014-11-610329
- Nature, 2014 516(7530):254-8
- Cancer Discov, 2014 4(9):1022-35
- Blood, 2014 123(2):239-50
- Blood, 2014 123(26):4120-31
- Nature, 2013 25;496(7446):523-7.
- Blood, 2012 120(19):3978-85
- Cancer Immunol Res, 2024 10.1158/2326-6066.CIR-23-1092
- Cell Rep, 2024 43(12):115061
- iScience, 2024 27(10):110939
- Transl Oncol, 2024 43:101857
- J Cell Commun Signal, 2024 18(1):e12017
- Nat Commun, 2023 14(1):1171
- Nat Commun, 2023 14(1):2935
- Mol Cell Proteomics, 2023 S1535-9476(23)00001-4
- Liver Int, 2023 10.1111/liv.15751
- Exp Neurol, 2023 363:114348
- Am J Cancer Res, 2023 13(2):452-463
- Cancers (Basel), 2023 15(9)2660
- Cancers (Basel), 2023 15(8)2373
- Cancers (Basel), 2023 15(3)671
- Sci Rep, 2023 13(1):3793
- Sci Rep, 2023 13(1):16950
- Sci Rep, 2023 13(1):11596
- Clin Exp Med, 2023 10.1007/s10238-023-01227-6
- Dis Model Mech, 2023 16(3)dmm049841
- J Clin Med, 2023 12(2)399
- Yonsei Med J, 2023 64(2):139-147
- Nat Cancer, 2023 10.1038/s43018-023-00635-7
- Nat Commun, 2022 13(1):6226
- Nat Commun, 2022 13(1):182
- Clin Cancer Res, 2022 28-20:4444-4455
- Leukemia, 2022 10.1038/s41375-022-01595-0
- Haematologica, 2022 10.3324/haematol.2021.279957
- Mol Syst Biol, 2022 18(8):e10855
- Cell Death Dis, 2022 13-9:755
- Cell Rep, 2022 41(6):111614
- Sci Signal, 2022 15(743):eabl9169
- Front Immunol, 2022 13:831680
- Hemasphere, 2022 6(6):e722
- Cancer Gene Ther, 2022 10.1038/s41417-022-00491-0
- Cell Biol Toxicol, 2022 10.1007/s10565-021-09670-5
- Commun Biol, 2022 5(1):740
- Mol Ther Oncolytics, 2022 26:105-119
- Front Pharmacol, 2022 13:1032975
- J Pers Med, 2022 12(2)258
- J Biol Chem, 2022 S0021-9258(22)01309-6
- Molecules, 2022 27(9)2742
- J Immunol, 2022 209(4):660-664
- Hum Cell, 2022 10.1007/s13577-022-00671-y
- BMC Cancer, 2022 22(1):1193
- PLoS One, 2022 17(11):e0277893
- J Radiat Res, 2022 rrac018
- Mol Genet Genomic Med, 2022 e2106.
- Mol Biomed, 2022 3(1):2
- Explor Target Antitumor Ther, 2022 3:37-49
- Mol Cell, 2021 81(4):708-723.e5
- J Exp Med, 2021 218(2)e20200517
- J Exp Med, 2021 218(12)e20211035
- J Allergy Clin Immunol, 2021 S0091-6749(21)00979-9
- Leukemia, 2021 10.1038/s41375-021-01326-x
- J Immunother Cancer, 2021 9(8)e002279
- Haematologica, 2021 10.3324/haematol.2021.278644
- Cancer Lett, 2021 S0304-3835(21)00532-2
- Cell Rep, 2021 37(2):109804
- Oncogene, 2021 10.1038/s41388-021-02049-0
- Blood Adv, 2021 5(10):2467-2480
- Blood Adv, 2021 5(23):5060-5071
- Front Immunol, 2021 12:745873
- Sci Signal, 2021 14(693)eabc1940
- Commun Biol, 2021 4(1):1333
- Cancer Immunol Immunother, 2021 10.1007/s00262-021-02988-3
- Cancers (Basel), 2021 13(12)2883
- J Immunol, 2021 207(5):1401-1410
- Hum Cell, 2021 34(6):1911-1918
- Hum Cell, 2021 10.1007/s13577-021-00579-z
- Hum Cell, 2021 10.1007/s13577-021-00639-4
- Cell Biol Int, 2021 10.1002/cbin.11557
- Clin Rheumatol, 2021 10.1007/s10067-021-05765-w
- J Clin Invest, 2020 19 pii: 134424
- Nucleic Acids Res, 2020 26;gkaa431
- Mol-Ther, 2020 6;28-5-:1263-1275
- J Exp Clin Cancer Res, 2020 39(1):228
- Haematologica, 2020 105(11):2584-2591
- Cancer Immunol Res, 2020 8(12):1532-1541
- Haematologica, 2020 10.3324/haematol.2019.227215
- Br J Cancer, 2020 10.1038/s41416-020-01117-8
- Br J Cancer, 2020 18
- Arterioscler Thromb Vasc Biol, 2020 40(1):86-102
- Oncogene, 2020 10.1038/s41388-020-1183-x
- Blood Adv, 2020 4(24):6106-6116
- Blood Adv, 2020 4(13):3072-3084
- Blood Adv, 2020 4(18):4382-4392
- Front Immunol, 2020 11:610523
- Cell Oncol (Dordr), 2020 8
- Br J Haematol, 2020 10.1111/bjh.17139
- Cancer Cell Int, 2020 19;20:58
- Cancer Cell Int, 2020 20:390
- Cancer Immunol Immunother, 2020 69(4):501-512
- Mol Cancer Ther, 2020 molcanther.0973.2019
- Eur J Immunol, 2020 8
- Mol Cancer Res, 2020 11
- Cancers (Basel), 2020 12(10)E2725
- J Gerontol A Biol Sci Med Sci, 2020 glaa216
- Viruses, 2020 12(6):E652
- Sci Rep, 2020 1;10(1):8867
- Hum Cell, 2020 10.1007/s13577-020-00420-z
- Hum Cell, 2020 10.1007/s13577-020-00425-8
- Int J Biochem Cell Biol, 2020 122:105734
- J Cell Sci, 2020 11 pii: jcs
- Cell Biol Int, 2020 10.1002/cbin.11322
- Turk J Haematol, 2020 37(3):167-176
- Iran J Pharm Res, 2020 19(1):153-165
- Data Brief, 2020 32:106185
- Clin Cancer Res, 2019 10.1158/1078-0432.CCR-19-2202
- Leukemia, 2019 10.1038/s41375-019-0486-9
- Leukemia, 2019 10.1038/s41375-019-0569-7
- Haematologica, 2019 10.3324/haematol.2019.216218
- Cell Death Dis, 2019
- Am J Transplant, 2019 19(5):1305-1314
- Blood Adv, 2019 3(20):2920-2933
- Breast Cancer Res, 2019 21(1):20
- Arch Pharm Res, 2019 10.1007/s12272-019-01163-8
- Int J Cancer, 2019 145(5):1312-1324
- Cell Biol Toxicol, 2019 10.1007/s10565-019-09487-3
- Cancer Sci, 2019 110(3):997-1011
- Mar Drugs, 2019 17(9)E526
- J Cell Mol Med, 2019 23(2):920-933
- Front Microbiol, 2019
- Cancers (Basel), 2019 11(10)
- Eur J Pharmacol, 2019 842:89-98
- Lab Invest, 2019 99(5):648-658
- J Immunol, 2019 202(5):1397-1405
- Int J Biochem Cell Biol, 2019 116:105615
- J Cell Biochem, 2019 120(8):14004-14016
- Toxicol Appl Pharmacol, 2019 381:114729
- Epigenetics, 2019 1-16
- Iran J Pharm Res, 2019 18(Suppl1):119-131
- Clin Cancer Res, 2018 24(1):120-129
- Clin Cancer Res, 2018 24(5):1103-1113
- Clin Cancer Res, 2018 24(16):3967-3980
- EMBO J, 2018 37(11)
- EBioMedicine, 2018 31:217-225
- Cancer Immunol Res, 2018 6(10):1150-1160
- Cell Death Dis, 2018 9(10):935
- Cell Death Dis, 2018 9(12):1160
- Cell Rep, 2018 22(4):860-868
- Cell Rep, 2018 24(8):2155-2166
- JCI Insight, 2018 3(11)
- Br J Haematol, 2018 182(5):654-669
- Br J Haematol, 2018 183(3):428-444
- Cancer Immunol Immunother, 2018 67(4):691-702
- Mol Cancer Ther, 2018 17(10):2091-2099
- J Cell Physiol, 2018 233(8):6052-6066
- Eur J Immunol, 2018 48(11):1796-1809
- Oncotarget, 2018
- Oncotarget, 2018 9(30):21166-21181
- Oncotarget, 2018 9(31):21820-21830
- Oncotarget, 2018 9(10):9273-9284
- Oncotarget, 2018 9(51):29565-29573
- Oncotarget, 2018 9(40):26019-26031
- Invest Ophthalmol Vis Sci, 2018 59(11):4477-4485
- J Immunol, 2018 201(2):406-416
- Ann Hematol, 2018 97(5):839-849
- Exp Eye Res, 2018 172:10-20
- Eur J Haematol, 2018 10.1111/ejh.13075
- Nat Commun, 2017 8(1):1090
- Nat Commun, 2017 8(1):2103
- Clin Cancer Res, 2017 23(9):2313-2324
- Cancer Res, 2017 77(8):1892-1904
- Proc Natl Acad Sci U S A, 2017 114(7):E1072-E1080
- Proc Natl Acad Sci U S A, 2017 114(6):E980-E989
- JCI Insight, 2017
- Oncogene, 2017 36(26):3651-3660
- Front Immunol, 2017 8:1221
- Arch Toxicol, 2017 91(8):2921-2938
- Mol Cancer Ther, 2017 16(11):2586-2597
- Mol Cancer Ther, 2017 16(9):1779-1790
- Oncotarget, 2017
- Oncotarget, 2017 8(4):6102-6113
- Oncotarget, 2017 8(14):23213-23227
- Oncotarget, 2017
- Oncotarget, 2017 8(70):114924-114934
- Oncotarget, 2017 8(47):81794-81802
- Oncotarget, 2017 8(1):742-756
- J Antimicrob Chemother, 2017 72(2):467-477
- Int J Oncol, 2017
- Int J Biochem Cell Biol, 2017 85:149-158
- Int J Biochem Cell Biol, 2017 91(Pt A):1-8
- PLoS One, 2017 12(3):e0172858
- Am J Reprod Immunol, 2017 78(4)
- J Pharmacol Sci, 2017 134(4):197-202
- Biochem Biophys Res Commun, 2017 491(4):939-945
- Anticancer Drugs, 2017 28(4):436-445
- Clin Cancer Res, 2016 10.1158/1078-0432.CCR-15-2242
- Leukemia, 2016 30(1):74-85
- Leukemia, 2016 30(2):337-45
- Leukemia, 2016 30(9):1963
- J Exp Clin Cancer Res, 2016 35(1):78
- Acta Pharmacol Sin, 2016 37(10):1281-1297
- Pain, 2016 157(1):137-46
- Br J Pharmacol, 2016 173(18):2726-38
- Thromb Haemost, 2016 115(6):1138-46
- Cancer Sci, 2016
- J Virol, 2016 90(14):6443-52
- Oncotarget, 2016 7(50):83424-83436
- Oncotarget, 2016 7(27):41031-41046
- Oncotarget, 2016
- Oncotarget, 2016 7(45):72608-72621
- Int J Oncol, 2016 48(1):253-60
- J Immunol, 2016 197(7):2918-29
- Oncol Rep, 2016 36(6):3643-3650
- Nat Commun, 2015 6:5937
- Clin Cancer Res, 2015 21(7):1628-38
- Proc Natl Acad Sci U S A, 2015 112(52):E7230-8
- Haematologica, 2015
- Haematologica, 2015 100(1):77-86
- Cell Death Dis, 2015 6:e1593
- Cell Rep, 2015 10.1016/j.celrep.2015.01.002
- Br J Haematol, 2015 171(5):726-35
- Int J Cancer, 2015 10.1002/ijc.29579
- J Leukoc Biol, 2015 98(6):1091-105
- Cancer Biol Med, 2015 12(4):401-8
- Oncotarget, 2015 6(42):44832-48
- Oncotarget, 2015 6(12):10399-414
- Oncotarget, 2015
- Stem Cells, 2015 10.1002/stem.1986
- J Immunol, 2015 194(5):2439-46
- PLoS One, 2015 10(9):e0137917
- Anticancer Res, 2015 35(5):2699-708
- J Clin Invest, 2014 124(4):1794-809
- Cell Death Differ, 2014 21(10):1535-45
- Clin Cancer Res, 2014 20(6):1576-89
- Pain, 2014 155(6):1150-60
- Br J Haematol, 2014 166(4):529-39
- J Immunol, 2014 192(5):2063-70
- PLoS One, 2014 9(6):e98818
- Chem Biol, 2014 21(8):955-66
- Leukemia, 2013 28(3):629-41
- Leukemia, 2013 27(10):2096-100
- Leukemia, 2013 27(10):2094-6
- Haematologica, 2013 98-1:57-64
- Cell Death Dis, 2013 4:e827
- Acta Pharmacol Sin, 2013 34(9):1201-7
- PLoS One, 2013 8(2):e57809
- PLoS One, 2013 8(8):e69126
- J Pharmacol Exp Ther, 2013 347(3):669-80
- Leuk Res, 2013 38(1):109-15
- Am J Pathol, 2012 180(5):1906-16
- PLoS One, 2012 7(7):e40852
- Eur J Cancer, 2011 48(1):149-57
化学信息&溶解度
| 分子量 | 415.42 |
| 分子式 | C22H18FN7O |
| CAS号 | 870281-82-6 |
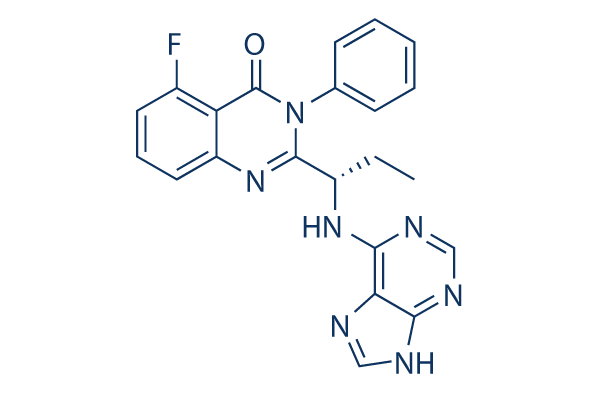
| Smiles | CCC(C1=NC2=C(C(=CC=C2)F)C(=O)N1C3=CC=CC=C3)NC4=NC=NC5=C4NC=N5 |
| 储存条件(自收到货起) | |
建议分装储备液,避免反复冻融! |
|
| 体外溶解度 | 批次: |
|
DMSO : 83 mg/mL ( 199.79 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 100 mg/mL ( 240.72 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 34 mg/mL Water : Insoluble DMSO : 83 mg/mL ( 199.79 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 23 mg/mL Water : Insoluble DMSO : 83 mg/mL ( 199.79 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 83 mg/mL Water : Insoluble |
|
| 体内溶解度 | 批次: |
| 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 | |
摩尔浓度计算器
质量(g)= 浓度(mol/L)* 体积(L)* 分子量(g/mol)
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入 μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
体内配方配制方法:取 μL DMSO母液,加入 μL Corn oil,混匀澄清。
注意:
1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。

