Fexagratinib (AZD4547)
别名: ABSK 091
目录号:S2801 Purity: 99.94%
Fexagratinib (AZD4547,ABSK 091)是一种新型选择性的FGFR抑制剂,靶向作用于FGFR1/2/3,在无细胞试验中IC50为0.2 nM/2.5 nM/1.8 nM,对FGFR4, VEGFR2(KDR)具有微弱的作用活性,对IGFR, CDK2和p38几乎没有作用活性。Phase 2/3。
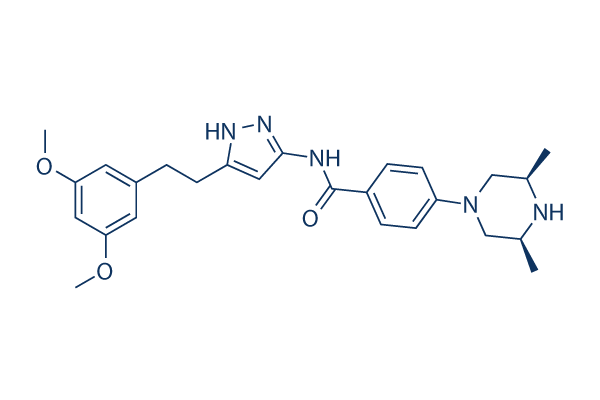
CAS: 1035270-39-3
客户使用Selleck的Fexagratinib (AZD4547) 发表文献138篇
- Cancer Discov, 2023 13(9):1998-2011
- Cancer Cell, 2022 S1535-6108(21)00662-0
- Nat Cancer, 2022 3(3):318-336
- Nature, 2021 595(7869):730-734
- Cancer Cell, 2019 35(3):504-518
- Nature, 2018 553(7686):101-105
- Science, 2017 10.1126/science.aan4368
- Nature, 2016 30;534(7609):647-51.
- Anticancer Drugs, 2025 36(2):97-103
- Acta Pharm Sin B, 2024 14(4):1693-1710
- Clin Cancer Res, 2024 10.1158/1078-0432.CCR-24-1834
- Cell Death Dis, 2024 15(1):64
- JCI Insight, 2024 9(15)e174888
- Cell Mol Life Sci, 2024 81(1):471
- Breast Cancer Res, 2024 26(1):54
- Br J Pharmacol, 2024 10.1111/bph.17341
- Clin Proteomics, 2024 21(1):3
- World J Oncol, 2024 15(3):492-505
- Acta Pharmaceutica Sinica B, 2024 10.1016/j.apsb.2024.01.013
- World J Oncol, 2024 15(2):192-208
- Cancer Res, 2023 83(19):3237-3251
- Cell Death Dis, 2023 14(7):463
- Br J Cancer, 2023 128(12):2186-2196
- Cancers (Basel), 2023 15(22)5354
- Front Oncol, 2023 13:1088444
- PLoS One, 2023 18(2):e0277316
- J Mammary Gland Biol Neoplasia, 2023 28(1):9
- Biol Open, 2023 12(8)bio059941
- Medicina (Kaunas), 2023 59(9)1635
- Nat Commun, 2022 13(1):2500
- Theranostics, 2022 12(14):6395-6408
- Cell Death Dis, 2022 13(8):724
- Oncogene, 2022 10.1038/s41388-022-02513-5
- JCI Insight, 2022 e157874
- NPJ Precis Oncol, 2022 6(1):52
- Oncoimmunology, 2022 11(1):2021619
- Mol Oncol, 2022 16(15):2823-2842
- Nephrol Dial Transplant, 2022 gfac220
- iScience, 2022 25(10):105182
- Cancer Sci, 2022 113(11:3888-3900)
- Int J Mol Sci, 2022 23(2)775
- Cancers (Basel), 2022 14(2)278
- J Biol Chem, 2022 S0021-9258(22)01172-3
- Development, 2022 dev.196220
- Dis Model Mech, 2022 15(8)dmm049611
- Heliyon, 2022 8(12):e12570
- Nat Commun, 2021 12(1):6572
- Acta Neuropathol, 2021 10.1007/s00401-021-02327-x
- Clin Cancer Res, 2021 27(9):2533-2548
- Cancer Res, 2021 canres.2276.2020
- Cancer Res, 2021 canres.4048.2020
- Proc Natl Acad Sci U S A, 2021 118(20)e2100342118
- EMBO Mol Med, 2021 13(9):e13193
- Breast Cancer Res, 2021 23(1):82
- J Transl Med, 2021 19(1):401
- Immunology, 2021 164(3):507-523
- Cells, 2021 10(6)1318
- Front Cell Dev Biol, 2021 9:758400
- Int J Oncol, 2021 10.3892/ijo.2021.5167
- FASEB J, 2021 35(2):e21286
- Sci Adv, 2021 7(7)eabd2645
- Genome Med, 2020 18;12(1):17
- EMBO Mol Med, 2020 e11793
- Proc Natl Acad Sci U S A, 2020 117(46):29025-29034
- EBioMedicine, 2020 62:103131
- Cancer Immunol Res, 2020 8(12):1542-1553
- British Journal of Cancer, 2020 1000–1011
- Br J Cancer, 2020 22
- Br J Cancer, 2020 10.1038/s41416-020-0952-1
- Acta Pharmacol Sin, 2020 10.1038/s41401-020-00567-3
- Stem Cell Res Ther, 2020 11(1):67
- Cell Oncol (Dordr), 2020 10.1007/s13402-020-00562-0
- Int J Mol Sci, 2020 18;21(8):2825
- Mol Cancer Res, 2020 18(4):549-559
- Cancers (Basel), 2020 12(9)E2697
- Front Oncol, 2020 10;10:331
- Oncol Rep, 2020 44(6):2581-2594
- BMC Pharmacol Toxicol, 2020 21(1):61
- Biochem Genet, 2020 10.1007/s10528-020-09982-x
- Nat Commun, 2019 10(1):2701
- J Exp Clin Cancer Res, 2019 38(1):372
- EMBO Mol Med, 2019 11(2)
- Cell Rep, 2019 28(9):2331-2344
- Front Pharmacol, 2019
- J Cell Mol Med, 2019 23(4):2933-2942
- J Cell Mol Med, 2019 101111/jcmm14793
- Mol Cancer Res, 2019
- Aging (Albany NY), 2019 11(18):7780-7795
- Transl Oncol, 2019 12(3):432-440
- Sci Rep, 2019 9(1):8726
- Sci Rep, 2019 9(1):1875
- Sci Rep, 2019 9(1):6004
- Ocul Immunol Inflamm, 2019 10.1080/09273948.2019.1672196
- Oncol Lett, 2019 18(6):6249-6260
- Reprod Sci, 2019 26(7):979-987
- Oncol Lett, 2019 17(2):2303-2307
- Hepatology, 2018 10.1002/hep.30127
- Cancer Lett, 2018 427:38-48
- Mol Cell Proteomics, 2018 17(5):850-870
- Oncogenesis, 2018 7(9):71
- Oncotarget, 2018 9(100):37352-37366
- J Biol Chem, 2018 293(38):14839-14849
- Sci Rep, 2018 8(1):12087
- Hum Mol Genet, 2018 27(6):1093-1105
- J Proteomics, 2018 170:130-140
- Theranostics, 2017 7(4):974-986
- Clin Cancer Res, 2017 23(4):962-973
- Clin Cancer Res, 2017 15;23(18):5527-5536
- Oncogene, 2017 10.1038/onc.2016.512
- Oncotarget, 2017
- Oncotarget, 2017 8(6):10007-10024
- Oncotarget, 2017 8(70):115596-115608
- BMC Cancer, 2017 17(1):191
- PLoS One, 2017 12(8):e0183181
- Mol Med Rep, 2017 15(3):1024-1030
- J Med Invest, 2017 64(3.4):262-265
- Clin Cancer Res, 2016 22(15):3884-93
- Cancer Res, 2016 76(11):3285-94
- Oncogene, 2016 10.1038/onc.2016.216
- Int J Cancer, 2016
- Oncotarget, 2016 7(31-:50161-50179
- Oncotarget, 2016 7(18-:25983-6002
- J Neurosci, 2016 36(16):4534-48
- J Proteome Res, 2016 15(12):4490-4504
- Hum Mol Genet, 2016 25(1):9-23
- Nat Commun, 2015 6:7002
- Mol Cancer Ther, 2015 14(10):2292-302
- Oncotarget, 2015
- J Biol Chem, 2015 290(44):26752-64
- PLoS One, 2015 10(6):e0123967
- PLoS One, 2015 10(4):e0124562
- Pathol Oncol Res, 2015 10.1007/s12253-015-9916-9
- Scand J Rheumatol, 2015 6:1-10
- Tumour Biol, 2015 10.1007/s13277-015-3237-1
- Gastric Cancer, 2014 10.1007/s10120-014-0444-1
- Mol Cell Biol, 2014 34(18):3535-45
- Cell Physiol Biochem, 2014 33(3):633-45
- Celon Pharma Inc, 2013 Paulina Grygielewicz
化学信息&溶解度
| 分子量 | 463.57 |
| 分子式 | C26H33N5O3 |
| CAS号 | 1035270-39-3 |
| Smiles | CC1CN(CC(N1)C)C2=CC=C(C=C2)C(=O)NC3=NNC(=C3)CCC4=CC(=CC(=C4)OC)OC |
| 储存条件(自收到货起) | |
建议分装储备液,避免反复冻融! |
|
| 体外溶解度 | 批次: |
|
DMSO : 93 mg/mL ( 200.61 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 93 mg/mL ( 200.61 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 93 mg/mL ( 200.61 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
|
| 体内溶解度 | 批次: |
| 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 | |
|
4%DMSO
30%PEG300
5%Tween80
61%ddH2O
浓度:5mg/ml
(10.79mM)
操作示例:以 1 mL 工作液为例,取40μL125mg/ml的澄清DMSO储备液加到300μL PEG300中,混合均匀使其澄清;向上述体系中加入50μLTween80,混合均匀使其澄清;然后继续加入610μL ddH2O定容至 1 mL。工作液请现配现用!
|
|
摩尔浓度计算器
质量(g)= 浓度(mol/L)* 体积(L)* 分子量(g/mol)
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入 μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
体内配方配制方法:取 μL DMSO母液,加入 μL Corn oil,混匀澄清。
注意:
1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。

