Tofacitinib
别名: CP-690550, Tasocitinib
中文名称:托法替尼,托法替布
目录号:S2789 Purity: 99.98%
Tofacitinib是一种新型JAK3抑制剂,在无细胞试验中IC50为1 nM,作用于JAK2和JAK1选择性低20到100倍。Tofacitinib 可在人类浆细胞样树突状细胞PDC中抑制抗凋亡的BCL-A1和BCL-XL并诱导PDC凋亡。
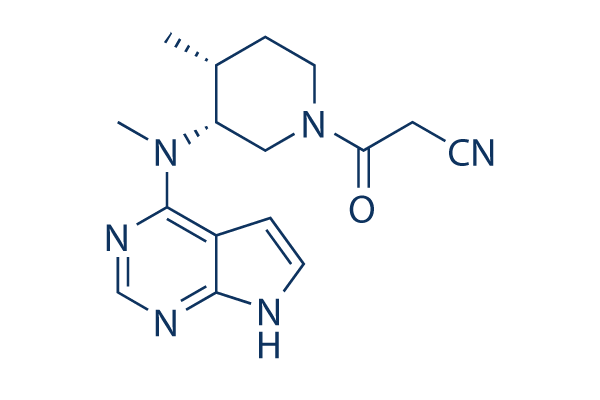
CAS: 477600-75-2
客户使用Selleck的Tofacitinib发表文献173篇
- Nature, 2024
- Nature, 2023 615(7951):305-314
- Sci Immunol, 2022 7(77):eabl9467
- Blood, 2022 blood.2021012366
- Cancer Discov, 2020 10(4):608-625
- Sci Immunol, 2019 4(40)
- Nat Med, 2017 23(3):291-300
- Immunity, 2017 21;46(2):220-232
- Nat Methods, 2016 13(2):151-7
- Ann Rheum Dis, 2016 75(1):311-5
- Blood, 2016 127(10):1287-96
- Blood, 2015 126(6):766-78.
- Blood, 2015 126(4):508-19
- Blood, 2014 124(5):761-70
- Blood, 2012 120(4):709-19
- Blood, 2011 118(14):3911-21
- Nat Commun, 2024 15(1):5873
- Cell Rep Med, 2024 S2666-3791(23)00615-8
- Cell Commun Signal, 2024 22(1):440
- NPJ Precis Oncol, 2024 8(1):152
- Elife, 2024 13e85914
- iScience, 2024 27(10):111049
- Mar Drugs, 2024 22(10)444
- Transl Oncol, 2024 44:101939
- Antimicrob Agents Chemother, 2024 68(4):e0135023.
- Arthritis Res Ther, 2024 26(1):181
- Arthritis Res Ther, 2024 26(1):178
- Eur J Pharm Sci, 2024 197:106767
- Molecules, 2024 29(8)1801
- Dis Model Mech, 2024 17(1)dmm050541
- Exp Dermatol, 2024 33(5):e15087
- Front Immunol, 2023 14:1187093
- Front Immunol, 2023 14:1078958
- Cells, 2023 12(5)770
- iScience, 2023 26(4):106309
- Clin Epigenetics, 2023 15(1):19
- Int J Mol Sci, 2023 24(9)7730
- bioRxiv, 2023 2023.09.22.559007
- medRxiv, 2023 10.1101/2023.12.01.23299102
- Nat Commun, 2022 13(1):6226
- J Clin Invest, 2022 132(11)e139298
- Nat Chem Biol, 2022 10.1038/s41589-022-01098-0
- J Immunother Cancer, 2022 10(1)e003766
- Haematologica, 2022 10.3324/haematol.2021.279848
- Front Immunol, 2022 13:773288
- Front Immunol, 2022 13:966067
- Front Immunol, 2022 13:960544
- Mol Oncol, 2022 16(20):3606-3619
- iScience, 2022 25(10):105182
- Arthritis Res Ther, 2022 24(1):266
- Clin Exp Immunol, 2022 uxac096
- Exp Dermatol, 2022 10.1111/exd.14566
- Nat Commun, 2021 12(1):5183
- Arthritis Rheumatol, 2021 10.1002/art.41666
- Clin Cancer Res, 2021 clincanres.1978.2021
- Cancer Lett, 2021 510:37-47
- Cell Chem Biol, 2021 S2451-9456(21)00303-2
- Int J Biol Macromol, 2021 185:907-916
- J Transl Med, 2021 19(1):165
- Anal Chem, 2021 10.1021/acs.analchem.0c03956
- Oncoimmunology, 2021 10(1):1943234
- RMD Open, 2021 7(3)e001737
- Cancers (Basel), 2021 13(16)3948
- Eur J Pharmacol, 2021 906:174214
- J Biol Chem, 2021 S0021-9258(21)01141-8
- J Biol Chem, 2021 297(3):101041
- Sci Rep, 2021 11(1):9561
- J Clin Med, 2021 10(7)1431
- Clin Exp Rheumatol, 2021 39 Suppl 129(2):161-170
- Autoimmunity, 2021 1-9
- Hum Mol Genet, 2021 ddab035
- Platelets, 2021 1-12
- J Immunotoxicol, 2021 18(1):154-162
- Exp Ther Med, 2021 22(4):1108
- J Immunol Methods, 2021 493:113020
- University of Tsukuba, 2021 10.3390/cancers13163948
- ACR Open Rheumatol, 2021 10.1002/acr2.11384
- Hepatology, 2020 25
- Leukemia, 2020 10.1038/s41375-020-01077-1
- J Neuroinflammation, 2020 29;17(1):170
- J Eur Acad Dermatol Venereol, 2020 34(4):800-809
- JCI Insight, 2020 5(4)
- Mol Cell Proteomics, 2020 10.1074/mcp.RA119.001504
- PLoS Pathog, 2020 16;16(3):e1008341
- Cells, 2020 9(7):1694
- Cells, 2020 9(9):E1927
- J Virol, 2020 JVI.01413-20
- Cancers (Basel), 2020 12(6):E1603
- Carcinogenesis, 2020 41(7):993-1004
- Clin Exp Immunol, 2020 201(3):233-243
- Onco Targets Ther, 2020 13:7165-7176
- J Clin Med, 2020 9(3)
- Exp Eye Res, 2020 S0014-4835(20)30611-4
- Mol Biol Rep, 2020 10
- ACR Open Rheumatol, 2020 2(1):3-10
- Nat Commun, 2019 10(1):4353
- Exp Mol Med, 2019 51(7):1-11
- J Thromb Haemost, 2019 10.1111/jth.14525
- Pharmacol Res, 2019 143:73-85
- Phytomedicine, 2019 59:152755
- Arthritis Res Ther, 2019 21(1):284
- Oncol Rep, 2019
- J Cancer Res Clin Oncol, 2019 145(2):411-427
- Immunotherapy, 2019 11(6):483-496
- J Orthop Res, 2019 37(4):972-980
- J Clin Invest, 2018 128(1):387-401
- Hepatology, 2018 67(4):1237-1252
- Allergy, 2018 73(9):1881-1891
- J Transl Med, 2018 16(1):156
- Sci Signal, 2018
- J Cell Sci, 2018 131(10)
- Clin Exp Rheumatol, 2018 36(4):559-567
- Ann Surg Oncol, 2018 25(8):2449-2456
- SLAS Discov, 2018 23(8):777-789
- Anticancer Res, 2018 38(4):1967-1977
- Nat Commun, 2017 1;8(1):1232
- Nat Chem Biol, 2017 13(1):38-45
- Proc Natl Acad Sci U S A, 2017 114(6):E980-E989
- JCI Insight, 2017
- Inflamm Res, 2017 66(1):25-37
- PLoS Pathog, 2017 13(12):e1006740
- Arch Toxicol, 2017 91(10):3385-3402
- Arch Toxicol, 2017 91(8):2921-2938
- Am J Pathol, 2017 187(5):980-986
- J Virol, 2017 91(9)
- Sci Rep, 2017 7(1):6779
- Sci Rep, 2017 7(1):10965
- Sci Rep, 2017 7:43441
- J Immunol, 2017
- J Immunol, 2017 198(2):657-668
- Biochem Biophys Res Commun, 2017 490(2):194-201
- Spine (Phila Pa 1976), 2017 42(14):E817-E824
- Oncol Lett, 2017
- Nat Chem Biol, 2016 12(5):373-9
- Leukemia, 2016 30(6):1311-9
- Sci Signal, 2016 9(456):ra116
- Oncoimmunology, 2016 5(3):e1094598
- Oncotarget, 2016 7(12):13886-901
- J Immunol Res, 2016 2016:5021537
- Transplant Proc, 2016 48(9):3046-3052
- Clin Cancer Drugs, 2016 3(2):131-137
- Mol Syst Biol, 2015 11(3):797
- J Med Chem, 2015 58(16):6589-606
- J Biol Chem, 2015 290(48):29022-34
- J Biol Chem, 2015 290(34):20972-83
- J Immunol, 2015 10.4049/jimmunol.1402739
- Prostate, 2015 10.1002/pros.22945
- Arthritis Rheumatol, 2014 66(1):121-9
- Arthritis Rheumatol, 2014 10.1002/art.39007
- Front Cell Neurosci, 2014 8:183
- Antimicrob Agents Chemother, 2014 58(4):1977-86
- Cell Signal, 2014 26(1):149-61
- PLoS One, 2014 9(1):e85463
- PLoS One, 2014 9(8):e103638
- PLoS One, 2014 9(11):e112014
- Biomed Res Int, 2014 2014:764981
- Mod Rheumatol, 2014 24(5):844-50
- Cancer Lett, 2013 341(2):224-30
- Transplantation, 2013 95(3):448-55
- J Cell Physiol, 2013 228(2):428-38
- J Immunol, 2013 192(1):349-57
- J Exp Med, 2012 209(2):259-73
- Genes Dev, 2012 26(19):2144-53
- J Infect Dis, 2012 205(11):1705-8
- J Cell Physiol, 2012 228(2):428-438
- J Biol Chem, 2012 287(20):16835-48
- J Biol Chem, 2012 287(41):34825-35
- J Immunol, 2012 189(6):2784-92
- Biochem Biophys Res Commun, 2012 418(2):234-40
- Int Immunopharmacol, 2011 11(12):2242-5
- Int Immunol, 2011 23(11):701-12
- Chem Biol, 2011 18(3):314-23
- Biochem Biophys Res Commun, 2010 402(3):500-6
化学信息&溶解度
| 分子量 | 312.37 |
| 分子式 | C16H20N6O |
| CAS号 | 477600-75-2 |
| Smiles | CC1CCN(CC1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N |
| 储存条件(自收到货起) | |
建议分装储备液,避免反复冻融! |
|
| 体外溶解度 | 批次: |
|
DMSO : 62 mg/mL ( 198.48 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 62 mg/mL ( 198.48 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 62 mg/mL ( 198.48 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 62 mg/mL ( 198.48 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble DMSO : 62 mg/mL ( 198.48 mM; DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
|
| 体内溶解度 | 批次: |
| 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 | |
|
5%DMSO
40%PEG300
5%Tween80
50%ddH2O
浓度:3.1mg/ml
(9.92mM)
操作示例:以 1 mL 工作液为例,取50μL62mg/ml的澄清DMSO储备液加到400μLPEG300中,混合均匀使其澄清;向上述体系中加入50μLTween80,混合均匀使其澄清;然后继续加入500μLddH2O定容至1mL。工作液请现配现用!
|
|
摩尔浓度计算器
质量(g)= 浓度(mol/L)* 体积(L)* 分子量(g/mol)
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入 μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
体内配方配制方法:取 μL DMSO母液,加入 μL Corn oil,混匀澄清。
注意:
1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。

